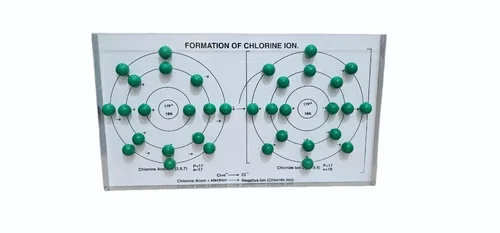
The Plastic Chemistry Chlorine Ion Model is an essential educational tool designed to simplify the understanding of ionic bonding, specifically the formation of the chlorine ion. Made from durable plastic, this 2D model is tailored for use in chemistry classrooms, science laboratories, and educational demonstrations targeting students from classes 9 to 12. With a focus on academic clarity and interactive learning, the model provides a clear representation of the electronic configuration and the transformation of a chlorine atom into a chloride ion. It serves as an effective aid for teachers and learners to visualize atomic structure, electron transfer, and the resulting ionic charge.
Description
Constructed with precision and attention to detail, the model demonstrates the outer shell of a chlorine atom, showing the seven valence electrons that make chlorine one electron short of a stable octet. It effectively captures the process in which chlorine gains one electron to complete its outer shell, thereby transforming into a negatively charged chloride ion (Cl⁻). The visual clarity of the model allows learners to understand how nonmetals like chlorine participate in ionic bonding by accepting electrons, particularly from metal atoms such as sodium, in the formation of common compounds like sodium chloride.
This 2D chlorine ion model is specifically designed to enhance comprehension by offering a hands-on and visual representation of atomic interactions that are otherwise abstract and theoretical. Whether used as part of a classroom lecture, laboratory session, or science fair project, it supports conceptual learning by bridging the gap between textbook information and real-world chemical behavior. It allows students to observe the step-by-step process of ion formation, which is critical in understanding more advanced concepts like electrovalent bonds, lattice structures, and conductivity in ionic compounds.
The material used in the model is high-quality, non-toxic plastic, which ensures longevity, reusability, and resistance to wear and tear from frequent handling in educational environments. It is lightweight and easy to carry, store, and clean, making it ideal for repetitive classroom use. The well-structured and polished surface of the model also makes it visually appealing and easy to interpret, even from a distance during classroom demonstrations.
Moreover, the model aligns with modern pedagogical strategies that emphasize active and visual learning. It promotes student engagement by encouraging inquiry, discussion, and hands-on interaction with theoretical topics. Teachers can use the model to prompt questions about the periodic table, electronegativity, ionization energy, and the chemical behavior of halogens. Students can collaborate in group learning settings, using the model to explain and reinforce their understanding of ion formation and its relevance in both inorganic and organic chemistry.
Ideal for both beginners and advanced learners, the Plastic Chemistry Chlorine Ion Model supports curriculum standards and helps learners build a strong foundation in chemical bonding. Its simplicity, durability, and academic relevance make it a valuable addition to any school or college science department. By offering a concrete representation of the formation of the chlorine ion, it enables learners to grasp complex chemical principles with greater ease and confidence.